| [Türkçe] | |
|
 |
| Turkish Society of Cardiology Young Cardiologists Bulletin Year: 4 Number: 1 / 2021 |
|
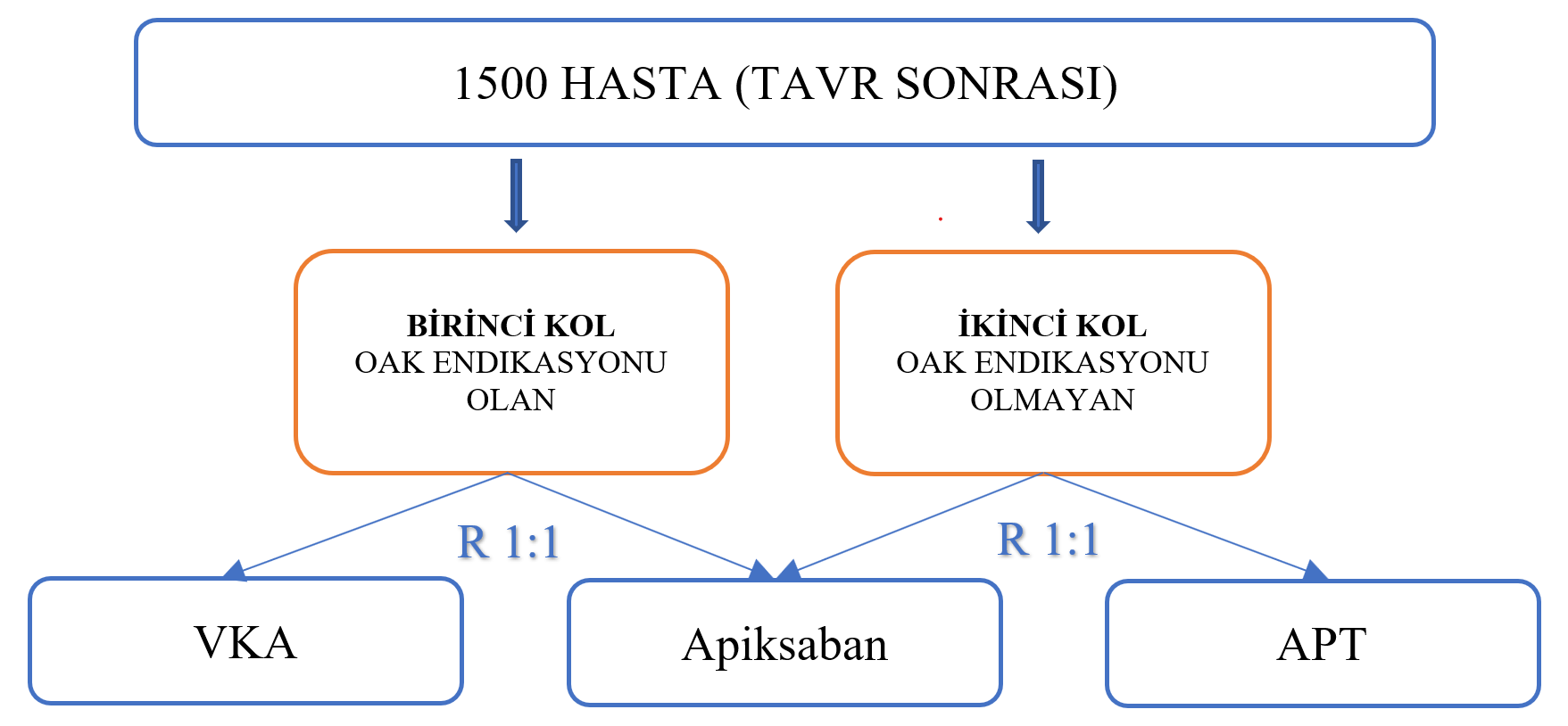
Reviwer: Dr. Alper Karakuş Name of the study : Anti-Thrombotic Strategy to Lower All cardiovascular and Neurologic Ischemic and Hemorrhagic Events after Trans-Aortic Valve Implantation for Aortic Stenosis ATLANTIS Presented Congress: May 15, ACC 21 Full Text Link: Background: There is no current evidence in the literature that new generation oral anticoagulants (OACs) can replace standard antiplatelet therapy (APT) or Vitamin K antagonists (VKA) after transcatheter aortic valve replacement (TAVR). However the GALILEO study showed more harm than benefit with low-dose rivaroxaban compared to APT. Objective: The aim of the study was to evaluate the efficacy and safety of apixaban 5 mg BID compared to standard APT or OAC therapy in patients undergoing TAVR. Method: 1500 patients who underwent successful TAVR were included in the study. Two different stratum were created according to OAK needs. The first stratum consisted of patients in need of OAC (n=451) and were randomized 1:1 to the VKA and apixaban groups. The second stratum consisted of the group that did not need OAC (n=1049) and the patients were randomized 1:1 to the APT and apixaban groups, All patients were followed for 12 months.
Results: The primary endpoint, time to death, stroke, myocardial infarction, systemic embolism, intracardiac or valve thrombosis, deep vein thrombosis/pulmonary embolism or major bleeding was 18.4% in the apixaban group and 20.1% in the standard therapy group (HR 0.92, 95% CI 0.73-1.16). In the post-Hoc sensitivity analysis, when valve thrombosis was not included in the composite endpoint, the composite outcome was 17.8% in the apixaban group versus 16.1% in the standard treatment group (HR 1.12, 95% CI 0.88-1.44). Bleeding, which is the primary safety outcome, was observed at a similar rate in both groups (8.5% vs. 8.5%). According to the results of valve evaluation with 4D computed tomography, the primary outcome (decreased motion [¾ grade] or hypo attenuated thrombosis [¾ grade] in >1 prosthetic leaflet) was 8.9% in the apixaban group and 13.0% in the standard treatment group at 90-day follow-up (p = 0.038). This rate was 5.5% vs. 9.5% (p = 0.28) in the first stratum apixaban group compared to VKA, and 15.9% vs. 8.7% in the second stratum apixaban group compared to APT (p = 0.011). Conclusion: The results show that apixaban is not superior to standard therapy (VKA if OAC is indicated; APT if not indicated) in patients undergoing TAVR. Leaflet thrombosis was found to be lower with apixaban compared to APT, but this did not show a benefit in clinical outcomes. Interpretation: Results from the ATLANTIS study were similar to the GALILEO study with low-dose rivaroxaban. Considering the concern of the mortality (non-cardiac) caused by use of apixaban, especially in patients without an indication for OAC, apixaban has not yet found a place in routine antithrombotic therapy after TAVR. However, the results of the study encourage the use of apixaban instead of VKA in patients requiring standard OAC therapy. |
| 2025 © Turkish Society of Cardiology. |