| [Türkçe] | |
|
 |
| Turkish Society of Cardiology Young Cardiologists Bulletin Year: 8 Number: 5 / 2025 |
|
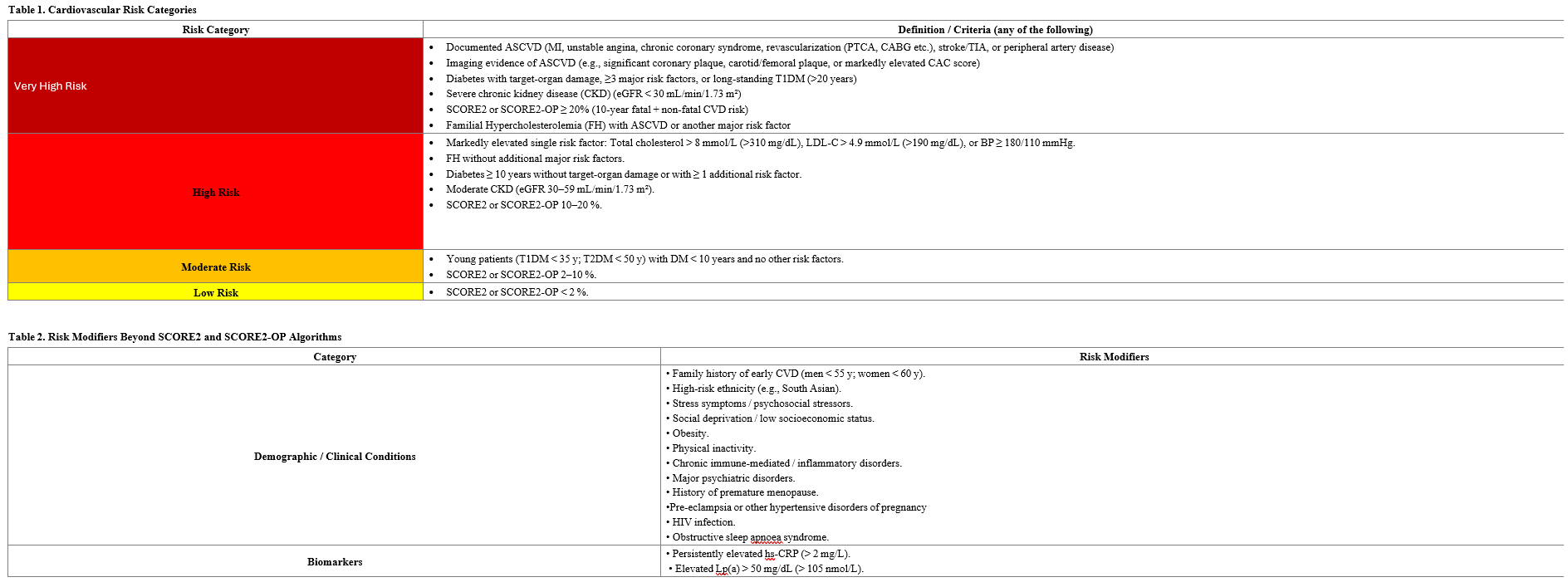
Written by Dr. Cemal Ozanalp Reference: Mach F, et al. 2025 Focused Update of the 2019 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J. 2025 Aug 29:ehaf190. doi: 10.1093/eurheartj/ehaf190. Introduction Since the publication of the 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias, numerous randomized controlled trials have provided new evidence guiding lipid‑lowering therapy. This focused update summarizes the new recommendations based on evidence available up to March 31, 2025. Atherosclerosis can be broadly described as a progressive inflammatory cascade triggered by the accumulation of low‑density lipoprotein cholesterol (LDL‑C) and other apolipoprotein B‑containing lipoproteins in the arterial wall, leading to plaque formation. The larger the plaque burden, the greater the risk of acute cardiovascular events. Thus, the primary goal remains the prevention of cardiovascular events by reducing plasma LDL‑C levels. In patients at higher cardiovascular (CV) risk, more intensive LDL‑C reduction should be achieved to reach the risk levels of lower‑risk individuals. Notably, patients of younger age derive greater absolute risk reduction from equivalent decreases in atherogenic lipids. Key Additions to the 2019 ESC/EAS Guidelines The Task Force has recommended the following major updates: 1- Use of the New SCORE2 and SCORE2‑OP Risk Estimation Algorithms As emphasized in the 2021 ESC Guidelines on Cardiovascular Disease Prevention, the burden of CV morbidity and mortality underscores the importance of lipid‑lowering therapy. Accordingly, the previous SCORE algorithm has been replaced by SCORE2 and SCORE2‑OP, which estimate the 10‑year risk of myocardial infarction, ischaemic stroke, and fatal CVD in adults aged 40–89 years without established ASCVD (Class I, Level B). Compared to the original SCORE model (which used total cholesterol and predicted only 10 years fatal CVD expectancy up to age 75), the SCORE2 and SCORE2‑OP algorithms incorporate HDL‑C and estimate both fatal and non‑fatal CVD risk up to age 89. These models are calibrated to four European country clusters—low, moderate, high, and very‑high CVD risk—based on national mortality data, as detailed in the 2021 ESC Prevention Guidelines. Importantly, LDL‑C treatment goals and therapeutic guidance across risk categories remain unchanged from the 2019 ESC/EAS Guidelines. Table 1 replaces the previous 2019 risk‑category table, allowing a more nuanced classification and earlier, more effective therapeutic planning. Table 2 lists risk modifiers to be considered beyond SCORE2 and SCORE2‑OP calculations. Primary Prevention Recommendations For patients without established ASCVD, pharmacologic lipid‑lowering therapy should be initiated when LDL‑C exceeds the following thresholds, in addition to lifestyle optimization: • Very‑high‑risk: LDL‑C ≥ 1.8 mmol/L (70 mg/dL) → initiate pharmacologic therapy (Class I, Level A) • High‑risk: LDL‑C ≥ 2.6 mmol/L (100 mg/dL) → initiate pharmacologic therapy (Class I, Level A) • Intermediate‑risk: LDL‑C ≥ 2.6 mmol/L (100 mg/dL) but >4.9 mmol/L (190 mg/dL) → consider therapy (Class IIa, Level A) • Low‑risk: LDL‑C ≥ 3.0 mmol/L (116 mg/dL) but >4.9 mmol/L (190 mg/dL) → consider therapy (Class IIa, Level A) In addition, subclinical coronary atherosclerosis—identified by imaging or elevated coronary artery calcium (CAC) score—should be treated as a risk‑enhancing factor, especially in intermediate‑risk individuals (Class IIa, Level B). 2- Lipid‑Lowering Therapies Targeting LDL‑C Reduction Bempedoic acid is an oral small‑molecule inhibitor of ATP‑citrate lyase, an enzyme acting upstream of HMG‑CoA reductase in the cholesterol‑synthesis pathway. It is a prodrug activated by very‑long‑chain acyl‑CoA synthetase 1, which is not expressed in skeletal muscle, explaining its low incidence of muscle‑related adverse effects (comparable to placebo). A daily dose of 180 mg reduces LDL‑C by ~23 % as monotherapy, ~18 % when added to statins, and up to ~38 % in fixed‑dose combination with ezetimibe. Treatment also lowers C‑reactive protein (CRP) levels without raising HbA1c in normoglycaemic or pre‑diabetic patients. Inclisiran is a small interfering RNA (siRNA) molecule that inhibits hepatic PCSK9 synthesis, offering an alternative to monoclonal antibodies (alirocumab, evolocumab). Phase III trials have demonstrated ~50 % LDL‑C reduction. Therapeutic Recommendations • Ezetimibe, proven to reduce cardiovascular events, should be used in statin‑intolerant patients to lower LDL‑C and ASCVD risk (Class I, Level A). • Bempedoic acid may be used to achieve LDL‑C goals in statin‑intolerant patients (Class I, Level B). • In high‑ or very‑high‑risk patients not achieving LDL‑C targets on maximally tolerated statins (with or without ezetimibe), bempedoic acid can be added to the therapy (Class IIa, Level C). • In patients aged ≥ 5 years with homozygous familial hypercholesterolaemia (HoFH) who fail to achieve LDL‑C targets on maximal therapy, evinacumab can be added (Class IIa, Level B). 3- Lipid‑Lowering Strategies in Acute Coronary Syndrome (ACS) - For hospitalized ACS patients, existing lipid‑lowering therapy should be intensified (Class I, Level C). - In treatment‑naïve ACS patients unlikely to reach LDL‑C goals with statin monotherapy, high‑intensity statin + ezetimibe combination therapy should be initiated during the index hospitalization (Class IIa, Level B). 4- Lipoprotein(a) [Lp(a)] Although Lp(a) is not yet included in SCORE2 or SCORE2‑OP algorithms, growing evidence supports its importance as an independent cardiovascular risk enhancer. RNA‑based injectable therapies (antisense oligonucleotides or siRNA agents) targeting apolipoprotein(a) synthesis in hepatocytes reduce Lp(a) levels by 80–98 %. An oral small‑molecule inhibitor and an additional siRNA agent capable of significantly lowering Lp(a) are under clinical investigation. Recommendations - In adults, Lp(a) > 50 mg/dL (105 nmol/L) should be considered a cardiovascular risk‑enhancing factor, with higher concentrations correlating with greater risk (Class IIa, Level B).< 5- Hypertriglyceridaemia Management Elevated triglycerides independently increase cardiovascular risk regardless of LDL‑C levels. Conventional fibrates (gemfibrozil, fenofibrate, bezafibrate) modestly lower triglycerides but have minimal impact on major adverse cardiovascular events (MACE). Volanesorsen, an antisense oligonucleotide targeting hepatic apolipoprotein C‑III mRNA, lowers plasma ApoC‑III, triglyceride, and chylomicron concentrations. Recommendations • In high‑ or very‑high‑risk patients with fasting triglycerides 135–499 mg/dL (1.52–5.63 mmol/L) despite statin therapy, adding high‑dose icosapent ethyl may be considered (Class IIa, Level B). • In severe hypertriglyceridaemia (>750 mg/dL or >8.5 mmol/L) due to familial chylomicronaemia syndrome, volanesorsen 300 mg weekly may be used to reduce triglycerides and pancreatitis risk (Class IIa, Level B). 6- Statin Therapy in HIV‑Positive Patients For adults aged ≥ 40 years living with HIV, statin therapy should be initiated regardless of baseline LDL‑C level or estimated risk, to reduce cardiovascular events (Class I, Level B). Drug interactions must be monitored carefully. 7- Statin Use in Oncology Patients at High/Very High Chemotherapy Induced Cardiotoxicity Risk Anthracycline‑based chemotherapy carries up to a 20 % risk of heart failure within five years, depending on cumulative dose. While non‑statin therapies remain under investigation, statins may reduce anthracycline‑induced cardiac dysfunction and should be considered in high‑ or very‑high‑risk cancer patients (Class IIa, Level B). 8- Dietary Supplements Use of nutritional supplements or vitamins lacking proven safety and LDL‑C‑lowering efficacy is not recommended for ASCVD risk reduction (Class III, Level B).
|
| 2026 © Turkish Society of Cardiology. |