|
Turkish Society of Cardiology
Young Cardiologists
President
Dr. Muzaffer Değertekin
Coordinator for the
Board of Directors
Dr. Ertuğrul Okuyan
Coordinator for the
Board of Directors
Dr. Can Yücel Karabay
Members
Dr. Adem Aktan
Dr. Gülşah Aktüre
Dr. Bayram Arslan
Dr. İnanç Artaç
Dr. Ahmet Oğuz Aslan
Dr. Görkem Ayhan
Dr. Ahmet Anıl Başkurt
Dr. Özkan Bekler
Dr. Oğuzhan Birdal
Dr. Yusuf Bozkurt Şahin
Dr. Serkan Bulgurluoğlu
Dr. Ümit Bulut
Dr. Veysi Can
Dr. Mustafa Candemir
Dr. Murat Çap
Dr. Göksel Çinier
Dr. Ali Çoner
Dr. Yusuf Demir
Dr. Ömer Furkan Demir
Dr. Murat Demirci
Dr. Ayşe İrem Demirtola Mammadli
Dr. Süleyman Çağan Efe
Dr. Mehmet Akif Erdöl
Dr. Kubilay Erselcan
Dr. Kerim Esenboğa
Dr. Duygu Genç
Dr. Kemal Göçer
Dr. Elif Güçlü
Dr. Arda Güler
Dr. Duygu İnan
Dr. Hasan Burak İşleyen
Dr. Muzaffer Kahyaoğlu
Dr. Sedat Kalkan
Dr. Yücel Kanal
Dr. Özkan Karaca
Dr. Ahmet Karaduman
Dr. Mustafa Karanfil
Dr. Ayhan Kol
Dr. Fatma Köksal
Dr. Mevlüt Serdar Kuyumcu
Dr. Yunus Emre Özbebek
Dr. Ahmet Özderya
Dr. Yasin Özen
Dr. Ayşenur Özkaya İbiş
Dr. Çağlar Özmen
Dr. Selvi Öztaş
Dr. Hasan Sarı
Dr. Serkan Sivri
Dr. Ali Uğur Soysal
Dr. Hüseyin Tezcan
Dr. Nazlı Turan
Dr. Berat Uğuz
Dr. Örsan Deniz Urgun
Dr. İdris Yakut
Dr. Mustafa Yenerçağ
Dr. Mehmet Fatih Yılmaz
Dr. Yakup Yiğit
Dr. Mehmet Murat Yiğitbaşı
Bulletin Editors
Dr. Muzaffer Değertekin
Dr. Can Yücel Karabay
Dr. Arda Güler
Contributors
Dr. Cemal Ozanalp
Dr. Emre Aydın
Dr. Kübra Okumuş
Dr. Merve Ortakaya
Dr. Muhammed Mustafa Yıldız
Dr. Süleyman Atalay
Dr. Şevval Kılıç
Dr. Veli Sonnur Şenlik
|
| |
|
|
|

New and Updated Recommendations in the ESC 2025 Guidelines for Pregnancy and Cardiovascular DiseasesTürk Kardiyoloji Derneði Genç Kardiyologlar Bülteni - New and Updated Recommendations in the ESC 2025 Guidelines for Pregnancy and Cardiovascular Diseases (Dr. Şevval Kılıç)New and Updated Recommendations in the ESC 2025 Guidelines for Pregnancy and Cardiovascular Diseases
Written by Dr. Şevval Kılıç
Reference:De Backer J, et al. 2025 ESC Guidelines for the management of cardiovascular disease and pregnancy. Eur Heart J. 2025. doi: 10.1093/eurheartj/ehaf193.
New recommendations
This new version of the guidelines not only includes an update to several recommendations but also introduces a structural revision. Let's take a look at the revisions:
1 The Pregnancy Heart Team
- Although the concept of the Pregnancy Heart Team was previously part of the general principles, it has now been given its own dedicated section, which covers all aspects from pre-conception through to the postpartum period.
- A discussion by the Pregnancy Heart Team about the high risk of maternal mortality or morbidity and the related high foetal risk is recommended for women with mWHO 2.0 class IV conditions, including a shared decision-making process for pregnancy termination, involving psychological support. (I,C)
- It is recommended that women with CVD of mWHO 2.0 class II–III and above are evaluated and managed by a Pregnancy Heart Team from pre-pregnancy onwards through pregnancy and post-partum. (I,C)
- Measurement of BNP and NT-proBNP levels should be considered prior to pregnancy in women with HF of any aetiology, including previous PPCM, cardiomyopathy, ACHD, and PAH, and be monitored during pregnancy according to the underlying disorder and in case of new-onset or worsening symptoms. (Iıa,B)
2) Drugs During Pregnancy and Lactation
- Given the importance of medication use throughout this document, this section has been brought forward and revised. DOACs are not recommended during pregnancy. (III,C)
3) Pregnancy in Women with Cardiomyopathies and Primary Arrhythmia Syndromes
- Delivery method: Vaginal delivery is recommended in most women with CMPs, unless there are obstetric indications for caesarean section, severe HF (EF <30% and/or NYHA class III/IV), uncontrolled arrhythmias, or severe outflow obstruction (≥50 mmHg) in women with HCM, or in women presenting in labour on VKAs. (I,C)
- In women with DCM and worsening of EF during pregnancy, counselling on the risk of recurrence during a subsequent pregnancy is recommended in all cases, even after recovery of LV function. (I,C)
- It is recommended that women with HCM with symptomatic LV dysfunction (EF <50%) and or severe LVOTO (≥50 mmHg) wishing to become pregnant are counselled by the Pregnancy Heart Team regarding the high risk of pregnancy-related adverse events. (I,C)
- Myosin inhibitors are not recommended in women during pregnancy due to lack of safety data. (III,C)
- Arrhythmias:
- Beta-blockers, with pre-pregnancy dose and with nadolol and propranolol as drugs of choice, are recommended during pregnancy and lactation in women with LQTS and CPVT. (I,B-C).
- It is recommended to continue beta-blocker therapy during lactation in women with LQTS to reduce arrhythmic risk.
- Pre-pregnancy dose beta-blockers of nadolol or propranolol is recommended in patients with LQT2, particularly in the post-partum period, which represents a high-risk period for life-threatening arrhythmias. (I,B)
- Flecainide, in addition to beta-blockers, is recommended in women with CPVT who experience cardiac events, such as syncope, VT, or cardiac arrest during pregnancy. (I,C)
4) Peripartum Cardiomyopathy
- Genetic counselling and testing should be considered in women with PPCM.
- When a reversible course of HF is assumed, treatment in accordance with HF guidelines should be considered for at least 12 months after complete LV recovery (normalization of LV volumes and EF). (IIA,C)
5) Pregnancy in Women with Aortopathies
- Since 2018, significant evidence has emerged in the context of heritable thoracic aortic disease (HTAD), supporting a more gene- and variant-based approach, which has been incorporated in this version of the guidelines.
- It is recommended that women with a history of aortic dissection or -surgery have pre-pregnancy counselling about the high risk by an extended Pregnancy Heart Team considering the presence and type of genetic variant, aortic morphology, growth rate, and aetiology of aortic dissection. (I,C)
6) Pregnancy in Women with Known Congenital Heart Disease
- It is recommended that all women with Fontan circulation who wish to become pregnant receive counselling from the Pregnancy Heart Team regarding the high risk of pregnancy-related adverse events. (I,C)
7) Pregnancy in Women with Pulmonary Arterial Hypertension
- It is recommended that women of childbearing potential with PAH wishing to become pregnant are counselled by a multidisciplinary team regarding the very high risk of pregnancy-related adverse events, encouraging a shared decision-making process about whether to become pregnant. (I,C)
8) Venous Thromboembolism in Pregnancy and Post-partum
- In pregnant women or women in the post-partum period with suspicion of venous thromboembolism (VTE) [deep vein thrombosis (DVT) and/or PE], an immediate formal diagnostic assessment with validated methods is recommended and should not be postponed. (I,B)
9) Recommendations for Coronary Artery Disease and Pregnancy
- In pregnant women with chest pain, it is recommended to exclude life-threatening cardiovascular conditions, including PE, ACS (including SCAD), and acute aortic syndrome. (I,C)
- The duration of DAPT (aspirin and clopidogrel) in pregnant women undergoing coronary stent implantation is recommended to be the same as in non-pregnant women, with an individual approach considering ischaemic risk and delivery-related bleeding risks. (I,C)
- Continuation of statins may be considered during pregnancy in women with established ASCVD. (Iıb,C)
10) Recommendations for Hypertensive Disorders and Pregnancy
- It is recommended to aim for systolic BP <140 mmHg and diastolic BP <90 mmHg in pregnant women. (I,B)
- In severe hypertension, drug treatment with i.v. Labetalol, urapidil, nicardipine, or oral short acting nifedipine or methyldopa is recommended for acute reduction in blood pressure. Intravenous hydralazine is a second-line option. (I,C)
11) Recommendations for Supraventricular Tachycardia and Pregnancy
- Therapeutic anticoagulation with LMWH is recommended for pregnant women with persistent or permanent AF at elevated thromboembolic risk. (I,C)
- Flecainide, in addition to beta-blockers, should be considered for long-term AF rhythm control in pregnancy. (Iıa,C)
12) Recommendation for Ventricular Tachycardia, Device Implantation and Catheter Ablation and Pregnancy
- When performing catheter ablation during pregnancy, the use of non-fluoroscopic mapping and navigation systems should be considered. (Iıa,C)
13) Recommendations for Cardiac Arrest and Pregnancy
- Continuous manual left uterine displacement during CPR in pregnant women (≥20 weeks) with cardiac arrest is recommended to relieve aortocaval compression. (I,C)
- It is recommended to establish i.v. Access above the diaphragm to ensure that the i.v. Therapy is not obstructed by the gravid uterus. (I,C)
- It is recommended that no drugs are withheld in pregnant women with cardiac arrest due to concerns of teratogenicity. (I,C)
14) Recommendation for congenital atrioventricular block and pregnancy
- In pregnant women with asymptomatic congenital AV block, normal cardiac anatomy and function, a narrow QRS complex, and ventricular rate (≥50 b.p.m.) a prophylactic temporary pacemaker during delivery is not recommended. (III,C)
15) Recommendation for Native Valve Disease and Pregnancy
- Valve surgery during pregnancy should only be considered when there is a maternal mortality risk and other treatment options have failed. (Iıa,C)
16) Recommendation for Prosthetic Valves Disease and Pregnancy
- It is recommended that a care plan documenting the agreed anticoagulant strategy (including the decision to continue VKAs or converting to therapeutic-dose LMWH in the first trimester) is in place for women of childbearing age with a MHV prior to pregnancy or as soon as pregnancy is recognized. (I,C)
17) Recommendations for Chronic and Acute Heart Failure and Pregnancy
- Inotropes and/or vasopressors are recommended in pregnant women with cardiogenic shock with levosimendan, dobutamine, and milrinone as recommended agents. (I,C)
- ACE-Is, ARBs, ARNIs, MRAs, ivabradine, and SGLT2 inhibitors are not recommended during pregnancy due to adverse foetal effects. (III,C)
18) Recommendations for Heart Transplantation and Pregnancy
- It is recommended to postpone pregnancy until at least 1 year after heart transplantation, taking individual risk factors into account. (I,C)
- In women with a heart transplant, it is recommended that immunosuppression serum drug levels are monitored during pregnancy every 4 weeks until the 32nd week, then every 2 weeks until the 36th week, then weekly until delivery and for 6–12 months after delivery to guide dosing. (I,C)
19) Recommendation for Cardio-Oncology and Pregnancy
- It is recommended that pregnant women with cancer who require cardiotoxic cancer therapy are jointly managed by the Pregnancy Heart Team and the cardio-oncology team. (I,C)
20) Long-term Effects of Adverse Pregnancy Outcomes
- This is a completely new section in the guidelines, reflecting the growing recognition of the importance of APOs.
- It is recommended to undertake a cardiovascular risk assessment in women with APOs, to recognize and document APOs when CVD risk is evaluated in women, and to provide counselling on the importance of healthy lifestyle choices that optimize cardiovascular health. (I,B)
Commentary:
Since the 2018 guideline, new evidence has emerged and clinical priorities have shifted, requiring an updated approach. Cardiovascular disease (CVD) remains a leading cause of maternal mortality and morbidity, and the new guideline aims to provide up-to-date, evidence-based recommendations to improve maternal outcomes—one of the WHO’s global health priorities. The 2025 version brings not only updated recommendations but also structural changes. The Pregnancy Heart Team is now a more formalized and central component, with the mWHO 2.0 classification guiding management, particularly in classes II–III and IV. Shared decision-making, psychosocial support, and patient autonomy are strongly emphasized, while heart failure management includes more specific parameters for monitoring and treatment.
For women with genetic or channelopathy-related arrhythmias, cardiomyopathies, or primary arrhythmia syndromes, pre-pregnancy counseling and multidisciplinary management are essential. The guideline shifts from rigid “pregnancy should be avoided” warnings toward a model of informed choice and shared decision-making, respecting patient values and life plans.
In heritable thoracic aortic disease (HTAD), there is greater focus on genetic testing and variant-specific risk assessment, moving beyond general recommendations to more refined, individualized risk stratification. Similarly, management of PAH and coronary artery disease in pregnancy is now more flexible and patient-centered, with treatment decisions based on overall condition, ischemic risk, and delivery-related factors rather than strict rules.
Management of hypertensive disorders now features clearer diagnostic thresholds, stronger multidisciplinary input, and earlier treatment initiation to reduce maternal-fetal complications.
Recommendations for SVT and VT are presented in structured sections (“acute,” “long-term,” “prevention”), and catheter ablation is recognized as a safe option for selected patients in experienced centers using fetal-safe techniques.
In heart failure, Levosimendan and Milrinone are now explicitly listed as recommended agents, while drugs with known fetal toxicity must be avoided. Post–heart transplant pregnancy is acknowledged as high-risk but manageable through standardized best practices and personalized multidisciplinary care. For women with cancer history, pre-pregnancy cardiovascular risk assessment is recommended, and Pregnancy Heart Teams specialized in cardio-oncology should oversee management, ensuring safe medication use and coordinated care.
Finally, adverse pregnancy outcomes (APOs)—such as pre-eclampsia, preterm birth, or low birth weight—are now recognized as long-term cardiovascular risk markers, not just pregnancy-specific complications. Women with prior APOs should undergo early and ongoing cardiovascular risk assessment and long-term follow-up to prevent future disease.
Overall, the 2025 ESC guideline represents a shift toward individualized, multidisciplinary, and patient-centered management, integrating shared decision-making, genetic insights, and long-term health perspectives into the care of women with heart disease.
Revised recommendations
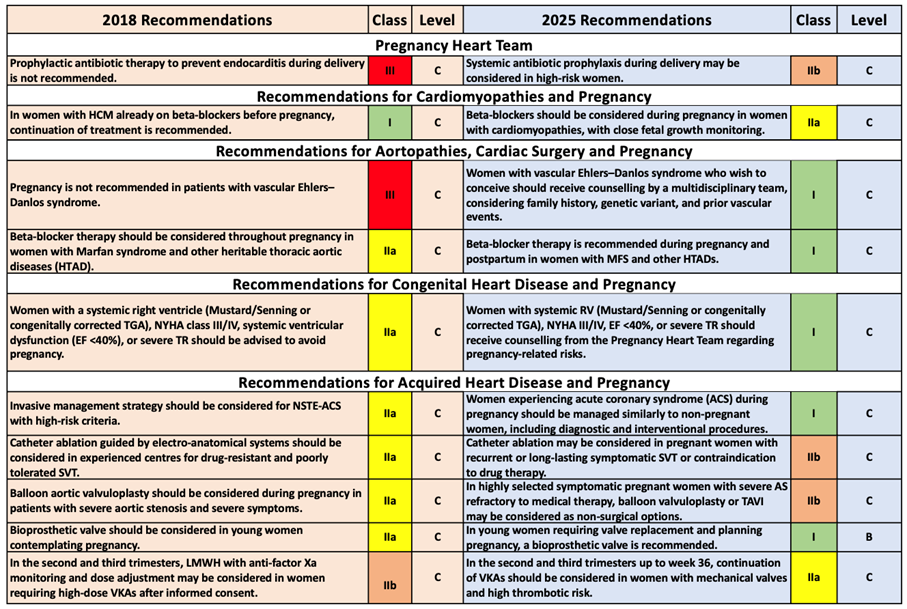
Interpretation:
The current recommendations in the ESC 2025 guideline indicate the adoption of a more proactive approach for women at risk of endocarditis during delivery. Furthermore, the 2025 guideline emphasizes women's reproductive rights, risk counseling, and the multidisciplinary team approach. Specifically, the definition of “high risk” must be clearly established—an internal protocol should be created regarding which women should receive this prophylaxis. Discussion within the team should take place for patients at “endocarditis risk” during pre-pregnancy and delivery planning.
The importance given to women's reproductive rights and individualized risk-benefit assessment has increased. The new guideline clearly emphasizes this approach. In such a situation, detailed pre-pregnancy counseling should be conducted with the woman and her partner; conditions should be set for genetic analysis, review of previous events, vascular status, and expert team evaluation. Furthermore, the use of beta-blockers in women with MFS (Marfan Syndrome) and HTAD (Hereditary Thoracic Aortic Disease) has become a stronger recommendation.
One of the most important changes in this guideline is the prominence given to risk management by respecting women's reproductive choices and highlighting shared decision-making processes with a multidisciplinary team. This reflects a contemporary approach that also considers risk tolerance and individual preferences. The Pregnancy Heart Team must be involved in pregnancy planning for women with high-risk congenital heart disease. Detailed pre-pregnancy evaluation, informing the patient and partner, and a risk-benefit agreement must be established.
For women with ACS (Acute Coronary Syndrome) in pregnancy, the approach to diagnosis and treatment (angiography, stenting, etc.) should be "standard approach under appropriate conditions" rather than "pregnancy = special treatment". It is important to prepare the team and the patient's family for this situation.
In the new ESC 2025 guideline, topics such as the woman's decision and informed consent, risk/benefit assessment, genetic counseling, and long-term effects have become much more prominent. The recommendation for women planning pregnancy to consult early with teams composed of disciplines such as cardiology, obstetrics, genetics, and anesthesiology has now become standard.

|


