|
Turkish Society of Cardiology
Young Cardiologists
President
Dr. Muzaffer Değertekin
Coordinator for the
Board of Directors
Dr. Ertuğrul Okuyan
Coordinator for the
Board of Directors
Dr. Can Yücel Karabay
Members
Dr. Adem Aktan
Dr. Gülşah Aktüre
Dr. Bayram Arslan
Dr. İnanç Artaç
Dr. Ahmet Oğuz Aslan
Dr. Görkem Ayhan
Dr. Ahmet Anıl Başkurt
Dr. Özkan Bekler
Dr. Oğuzhan Birdal
Dr. Yusuf Bozkurt Şahin
Dr. Serkan Bulgurluoğlu
Dr. Ümit Bulut
Dr. Veysi Can
Dr. Mustafa Candemir
Dr. Murat Çap
Dr. Göksel Çinier
Dr. Ali Çoner
Dr. Yusuf Demir
Dr. Ömer Furkan Demir
Dr. Murat Demirci
Dr. Ayşe İrem Demirtola Mammadli
Dr. Süleyman Çağan Efe
Dr. Mehmet Akif Erdöl
Dr. Kubilay Erselcan
Dr. Kerim Esenboğa
Dr. Duygu Genç
Dr. Kemal Göçer
Dr. Elif Güçlü
Dr. Arda Güler
Dr. Duygu İnan
Dr. Hasan Burak İşleyen
Dr. Muzaffer Kahyaoğlu
Dr. Sedat Kalkan
Dr. Yücel Kanal
Dr. Özkan Karaca
Dr. Ahmet Karaduman
Dr. Mustafa Karanfil
Dr. Ayhan Kol
Dr. Fatma Köksal
Dr. Mevlüt Serdar Kuyumcu
Dr. Yunus Emre Özbebek
Dr. Ahmet Özderya
Dr. Yasin Özen
Dr. Ayşenur Özkaya İbiş
Dr. Çağlar Özmen
Dr. Selvi Öztaş
Dr. Hasan Sarı
Dr. Serkan Sivri
Dr. Ali Uğur Soysal
Dr. Hüseyin Tezcan
Dr. Nazlı Turan
Dr. Berat Uğuz
Dr. Örsan Deniz Urgun
Dr. İdris Yakut
Dr. Mustafa Yenerçağ
Dr. Mehmet Fatih Yılmaz
Dr. Yakup Yiğit
Dr. Mehmet Murat Yiğitbaşı
Bulletin Editors
Dr. Muzaffer Değertekin
Dr. Can Yücel Karabay
Dr. Muzaffer Kahyaoğlu
Dr. Ahmet Karaduman
Contributors
Dr. Ahmet Anıl Başkurt
Dr. Ayşe Nur Özkaya İbiş
Dr. Cemalettin Yılmaz
Dr. Mahmut Buğrahan Çiçek
Dr. Mustafa Karanfil
Dr. Ömer Kümet
Dr. Özkan Bekler
Dr. Özkan Karaca
Dr. Seda Tanyeri Üzel
Dr. Yasin Özen
Dr. Yusuf Bozkurt Şahin
Dr. Yücel Kanal
|
| |
|
|
|

Randomised comparison of Abluminus DES+ sirolimus-eluting stents versus everolimus-eluting stents in coronary artery disease patients with diabetes mellitus global: the ABILITY diabetes global studyTürk Kardiyoloji Derneği Genç Kardiyologlar Bülteni - Randomised comparison of Abluminus DES+ sirolimus-eluting stents versus everolimus-eluting stents in coronary artery disease patients with diabetes mellitus global: the ABILITY diabetes global study (Dr. Mustafa Karanfil)Name of the Study: Randomised comparison of Abluminus DES+ sirolimus-eluting stents versus everolimus-eluting stents in coronary artery disease patients with diabetes mellitus global: the ABILITY diabetes global study
Published in congress: EuroPCR 2024
Dr. Mustafa Karanfil
Introduction:
Diabetes mellitus (DM), affecting 40% of coronary artery disease (CAD) patients, creates a prothrombotic and proinflammatory environment, thereby increasing the risk of ischemic complications. Patients with DM who undergo percutaneous coronary intervention (PCI) experience higher rates of major adverse cardiac events (MACE) and repeat revascularization. New-generation drug-eluting stents (DES) have replaced bare metal stents (BMS) and first-generation DES, but information on long-term clinical outcomes in diabetic patients is limited. Abluminus DES is a new stent designed for diabetic patients, with a thin-strut, cobalt-chromium structure releasing sirolimus.
Objectives:
This study aims to test the efficacy and safety of Abluminus DES compared to XIENCE stent, which releases everolimus, in diabetic patients with minimal exclusion criteria applied.
Methods:
A prospective, multicenter, multinational, randomized, open-label, 2-arm study was conducted, including type 1 and 2 DM patients with stable CAD or NSTE-ACS and excluding STEMI, cardiogenic shock, PCI for in-stent restenosis, or PCI for bypass grafts. The first co-primary endpoint was non-inferiority for target lesion revascularization (TLR) with an event rate of 7.5% and a non-inferiority margin of 3%. The second co-primary endpoint was non-inferiority for target lesion failure (TLF), including TLR, target vessel-related MI, and cardiovascular death, with an event rate of 9% and a non-inferiority margin of 3%.
Results:
A total of 3050 patients were randomized, with 1514 patients in the Abluminus DES arm and 1520 patients in the XIENCE DES arm included in the intention-to-treat (ITT) analysis. There were no differences in demographic, clinical, diabetic, or procedural characteristics between the groups. At 1 year, the rate of TLR was 2.14% in the XIENCE arm and 4.78% in the Abluminus arm, with a treatment difference of 2.64% (95% CI: 1.28-4.00), hazard ratio (95% CI): 2.27 (1.48-3.50), and a P-value for non-inferiority of 0.409. At 1 year, the rate of TLF was 6.25% in the XIENCE arm and 9.66% in the Abluminus arm, with a treatment difference of 3.41% (95% CI: 1.52-5.40), hazard ratio (95% CI): 1.58 (1.21-2.06), and a P-value for non-inferiority of 0.658.
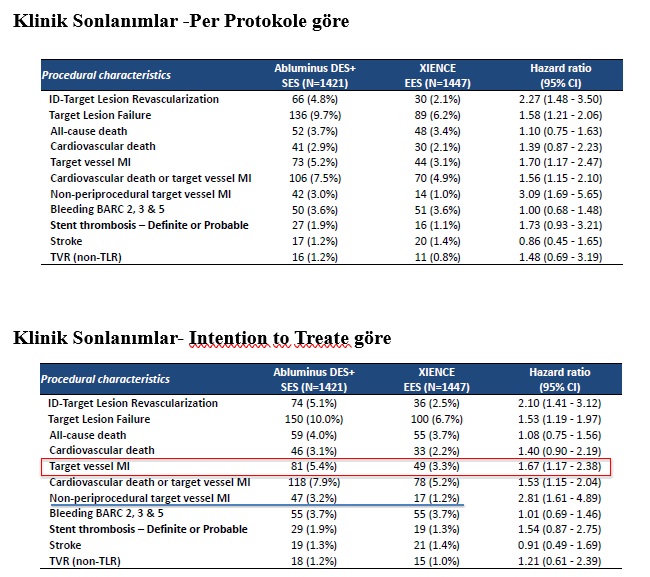
Conclusion:
The open-label design of the study, along with the novel implantation mechanism and long inflation time (45 seconds) of the Abluminus stent, as well as operators' unfamiliarity with it, and the unexpectedly low event rates in the control group may have influenced the results.
Comments:
The ABILITY DIABETES-GLOBAL study is the largest, head-to-head, randomized clinical trial comparing the new Abluminus DES to the XIENCE everolimus-eluting stent in moderate-risk diabetic patients undergoing PCI. Both stent platforms were safe and effective, and the clinical performance of Abluminus DES was in line with current-generation drug-eluting stents. Non-inferiority was not met for the two co-primary endpoints. However, these results may be due to lower than expected event rates in the control group. A 2-year follow-up is planned, and long-term results will provide further clarity on whether Abluminus DES implantation is associated with better clinical outcomes in diabetic patients.

|


