| [Türkçe] | |
|
 |
| Turkish Society of Cardiology Young Cardiologists Bulletin Year: 6 Number: 7 / 2023 |
|
Name of the Study: Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity reiwer : Dr. Başak Çatalbaş Kahraman Published Congress: ESC 2023 Link: Background: The prevalence of heart failure with preserved ejection fraction (HFpEF) is increasing, and it's associated with high symptom burden and functional impairment in obese individuals. There is no approved treatment targeting HFpEF associated with obesity. It's unclear whether pharmacotherapies targeting obesity can reduce symptoms, physical limitations, and improve exercise capacity in this patient group. Hence, this is the first study conducted in this area. Objective:Semaglutide is a potent GLP-1 receptor agonist approved for long-term weight management, showing significant weight loss and positive effects on cardiometabolic risk factors in overweight or obese individuals. This study aimed to investigate whether once-weekly 2.4 mg semaglutide could provide weight loss, reduce symptoms, physical limitations, and improve exercise capacity in HFpEF and obese patients. . Methods: In a 52-week randomized trial, 529 patients with HFpEF and a body mass index of 30 or higher were randomly assigned to receive once-weekly semaglutide (2.4 mg) or placebo. The primary endpoints were change from baseline in Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS; a score between 0 and 100, where higher scores indicate fewer symptoms and physical limitations) and change in body weight. Secondary endpoints included change in 6-minute walk distance; a composite endpoint of death, heart failure events, KCCQ-CSS, change in 6-minute walk distance, and change in CRP levels. A total of 2,989 patients who had primary PCI after STEMI at low-to-intermediate risk were randomly assigned to PPA (n=1,494) or placebo (n=1,495). And treated at least 48 hours after PCI. The primary efficacy endpoints were defined as all-cause death, non-fatal myocardial infarction (MI), non-fatal stroke, stent thrombosis, or urgent revascularization of any epicardial vessel within 30 days. The primary safety endpoint was BARC 3-5 major bleeding within 30 days. Results: Average change in KCCQ-CSS: Semaglutide: 16.6 points Placebo: 8.7 points (Estimated difference: 7.8 points; 95% CI: 4.8 to 10.9; p < 0.001) Average change in body weight: Semaglutide: -13.3% Placebo: -2.6% (Estimated difference: -10.7 points; 95% CI: -11.9 to -9.4; p < 0.001) Average change in 6-minute walk distance: Semaglutide: 21.5 meters Placebo: 1.2 meters (Estimated difference: 20.3 meters; 95% CI: 8.6 to 32.1; p < 0.001) Hierarchical composite endpoint analysis showed that semaglutide had superior gains compared to placebo (win ratio: 1.72; 95% CI: 0.51 to 0.72; p < 0.001) Average percent change in CRP levels: Semaglutide: -43.5% Placebo: -7.3% (Estimated treatment ratio: 0.61; 95% CI: 0.51 to 0.72; p< 0.001) Serious adverse events: 35 participants (13.3%) in semaglutide group and 71 participants (26.7%) in placebo group reported them. During the study, 7 participants died; 3 in the semaglutide group and 4 in the placebo group. The cause of one death in the placebo group was cardiovascular, and the cause of the other 6 deaths (3 in each group) was not cardiovascular. One death in each group was due to COVID-19. 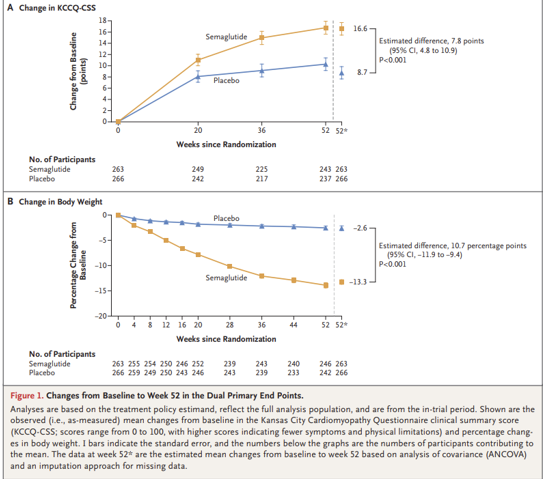
Conclusion: Semaglutide (2.4 mg) treatment in HFpEF and obese patients led to improvements in symptoms and physical limitations (measured by KCCQ-CSS), and exercise function compared to placebo. Interpretations: The results from the semaglutide study indicate that pharmacotherapies targeting obesity could be a new treatment modality for HFpEF patients associated with obesity, offering potential benefits in symptom reduction and improved exercise capacity. |
| 2025 © Turkish Society of Cardiology. |
