| [Türkçe] | |
|
 |
| Turkish Society of Cardiology Young Cardiologists Bulletin Year: 6 Number: 5 / 2023 |
|
One-Year Outcomes of the Pivotal Study of DragonFly Transcatheter Valve Repair System for Degenerative Mitral Regurgitation Reviewer: Dr. Elif Güçlü Congress:EUROPCR 2023 Introduction Transcatheter mitral valve intervention treatment is a promising alternative therapy for patients with severe mitral regurgitation(MR). This is a prospective , single arm, multicenter study in China, first-in human study of transcatheter edge-to-edge repair (TEER) using a novel device for severe MR. The device in our study, the first domestic transfemoral TEER device in China, has unique design features andis capable of treating both straight-forward and anatomically complex MR. Patients are clinically symptomatic patients with chronic moderate to severe (3+) or severe (4+) organic mitral regurgitation (DMR) who were assessed as high risk for surgical procedures by the cardiac team at the local clinical trial site. Subjects are enrolled and treated with the DragonFly Transcatheter Mitral Valve Repair System. All subjects receive clinical follow-up immediately after the procedure, before discharge, and 30 days, 6 months, and 12 months after the procedure. Treatment success at 12 months is used as the primary endpoint, with the definition as freedom from death, valve dysfunction surgery, and moderately severe or severe mitral regurgitation (MR >2+) at 12 months. The secondary endpoints include acute procedural success, acute device success, and surgery for valve dysfunction, NYHA class I or II at 30 days, 6 months, and 12 months, and the improvement in the quality of life change from baseline as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 12 months after the procedure. The safety endpoints include major adverse events (MAEs), all-cause mortality, and cardiac mortality at 30 days, 6 months, and 12 months after the procedure. To evaluate the safety and effectiveness of the Valgen Medtech DragonFly Transcatheter Mitral Valve Repair System in the treatment of patients with clinically significant chronic moderate (3+) or severe (4+) degenerative mitral regurgitation (DMR) who have been evaluated by the local heart team as being at high surgical risk, and to evaluate the product performance. Inclusion Criteria: * MR ≥3+* NYHA functional class ≤ II * LVEF ≥ %23. * Anatomically suitable assessed by site investigators and confirmed by the echo core laboratory and eligibility committee. * High surgical risk as defined by either STS Risk Calculator Exclusion Criteria: * Echocardiographic evidence of intra-cardiac mass, thrombus, or vegetation.* The presence of other severe heart valve disease requiring surgical intervention. * Mitral endocarditis, or rheumatic heart disease * Estimated pulmonary artery systolic pressure > 70 mmHg by echocardiography. * Cerebrovascular accident within 30 days prior to randomization or symptomatic severe carotid stenosis (>70% by ultrasound). Modified Rankin Scale ≥ 4. * Hemodynamic instability defined as systolic pressure < 90 mmHg without afterload reduction, cardiogenic shock, or the need for inotropic support or an intra-aortic balloon pump. 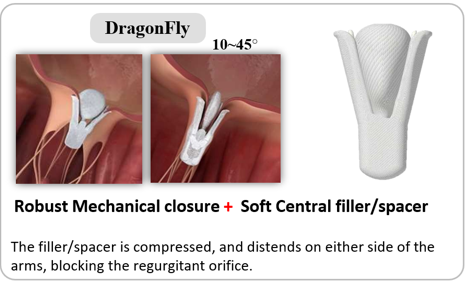
The device in our study has the following characteristics. 1 The device features a central, compressible filler, which makes it unique, as with closure ofthe grasping arms causes this filler to spread out to fill the residual regurgitant orifice. This can be accomplished with the grasping arms locking in any angles ranging from 0 to 45, which delivers a secure leaflet holding while decreasing the strain concentration on the clipping areas when dealing with small mitral valve orifice area or relatively short leaflet situations to avoid increasing transvalvular gradient after TEER procedure.2 The device comes in 4 sizes. 3 The delivery system includes a guide catheter and a steerable sleeve with readable reflection graduations on each handle. 4 The metal stabilizer with gear design on the rail and bracket knobs provides accurate and reliable forward and backward control of the clip delivery system. The device arms are closed simultaneously and mechanically locked (not self-locking) via the central compressible filler and the mechanical locking mechanism. |
| 2025 © Turkish Society of Cardiology. |
