|
Pulmoner Vasküler ve Erişkin Doğumsal Kalp Hastalıkları Çalışma Grubu
Yönetim Kurulu
Başkan:
Prof. Dr. Bahri Akdeniz
Y.K. adına Koordinatör:
Dr. Dursun Aras
Y.K. adına Koordinatör:
Dr. Bülent Mutlu
Üyeler
Halil Ataş
Mehmet Kaplan
Ergün Barış Kaya
Murat Meriç
Gülten Aydoğdu Taçoy
|
|
|
 
Olgu Sunumu.
PH Gündem - Balloon Pulmonary Angioplasty;
Review of BPA-Related Pulmonary Injury, Therapeutic Strategy
and Potantial Reasons (Sena SERT , Bedrettin YILDIZELI, Bulent MUTLU<br>
Marmara University, Faculty of Medicine, Department of Cardiology, Istanbul, Turkey)Balloon Pulmonary Angioplasty;
Review of BPA-Related Pulmonary Injury, Therapeutic Strategy
and Potantial Reasons
Sena SERT , Bedrettin YILDIZELI, Bulent MUTLU
Marmara University, Faculty of Medicine, Department of Cardiology, Istanbul, Turkey Introduction:
Chronic thromboembolic pulmonary hypertension (CTEPH) is a long-term consequence of acute/recurrent pulmonary thromboembolic disease (1,2). Pulmonary endarterectomy (PEA) is the first line treatment for CTEPH patients albeit depends on patient’s characteristics, co-morbidities and surgical accessibility. Some patients are not suitable candidates for PEA or persistent/recurrent pulmonary hypertension (PH) continues after PEA. Balloon pulmonary angioplasty (BPA) is an alternative approach that has been proven the efficacy on exercise capacity and hemodynamics with inoperabl / recurrent or residual CTEPH patients (1,2,3). Even though hemodynamic and exercise capacity improvement with BPA has demonstrated on CTEPH population, high rates of complications especially as a leading cause- reperfusion lung injury (RLI) remains as a challenging topic. In this review we will outline; what is the current concept to explain the underlying mechanism, what to do to prevent RLI complication (pre/peri- procedural care), how to recognize and how to manage it.
Clinical Presentation
31-year old woman has followed as inoperable CTEPH for 3 years. She was admitted to PH outpatient clinic with exertional dyspnea. World Health Organization (WHO) functional class was III despite riociguat 2,5 mg T.I.D., rivaroksaban 20 mg. Mild right ventricular (RV) overload fingings and hypoxia was detected. Blood pressure: 95/ 65 mmHg, heart rate: 120/ min, saturation at room air: %89. 6-minute walk test (6MWT): 330 m. NT-pro-BNP: 1500 ng/ml. Echocardiography: dilated right ventricle (RV:46*41*64mm), reduced RV ejection fraction: 40 %, RV fractional area change: 30 %. Right heart catheterization showed that mean pulmonary arterial hypertension (PAPm): 56 mm Hg; cardiac index (CI): 1,68 L/min/m2; pulmonary vascular resistance (PVR): 21 Wood Unit. Abrupt vascular narrowing and pouch lesions detected at both lobes in distal segments. She was assessed as inoperable by experienced surgeons cause of the location of the lesions and its surgical inaccessibility. Epoprostenol administered at 1 ng/kg/min by increasing 1 ng/kg/min each day till reaching the maximum of 5 ng/kg/min dose, 6 days before BPA.
Venous access was supplied via right common femoral vein. After selective angiography with 6-Fr guiding catheter; BPA planned to restricted for left lung to A5-9 segments. 0,014 guidewire advanced through the lesions at A5. We started balloon inflation with 1,5 mm monorail balloon than increased to 2 mm. BPA repeated 4 to 5 times until the balloon size reached maximum 90% of the original vessel diameter. Same steps repeated for A9 segment with same balloon size. During the manipulations at this site, the nature of the lesion obligated the operator to deeply engage into lesion site with the tip of the wire. After guidewire advanced through pouch like lesion, aware of the deep insertions before, balloon inflation has performed gently. Than we confirmed no contrast extravasation, no hemo-sputum, no exceeding hypoxia, no pulmonary changes with chest X-ray after BPA. But 20 minutes after the procedure, she had progressive tachypnea and desaturation. Reperfusion injury was confirmed with chest X-ray at left lower lung (Figure-2). Non-invasive positive pressure ventilation (NIPPV) has administered at intensive care unit. Reperfusion injury followed with computerized tomography (CT) images next days. Mechanical ventilation and percutaneous cardiopulmonary support has not been required. Lung injury and symptoms normalized at the end of the week. After BPA intervention her control echocardiography has compared the one before treatment (Figure-3), mPAP levels decreased about 40 mmHg. Her functional capacity regressed to WHO Class II, Six-minute walk distance increased to 500 m from 340 m. and brain natriuretic peptide levels decreased 70 pg/ml from 1500 pg/ml. Now we are planning to perform more sessions for untreated segments.
Discussion:
Inoperable CTEPH patients with mPAP> 30 mmHg whom medically-treated, has shown to have poor prognosis (4). 5-year survival has found approximately 60% with solely medical treatment (4). As an alternative treatment for PEA, BPA has first reported in 1988 (5). In 2001, Feinstein et al (6) has demonstrated hemodynamic and exercise capacity improvement with BPA on CTEPH population despite high rates of complications which was the reason of BPA ‘s unfavorable acceptance. Than Matsubara et al. (7) and some other groups reported BPA series of in-operable and peripheral type CTEPH patients with preferable results in hemodynamics and exercise capacity. However, complication rates remained high (approximately 60%) in comparison with PEA (16% to 22%) (7,8). The leading complication was RLI, also vascular complications observed as pulmonary artery branches perforation/ rupture/ dissection. With the light of the information obtained from PEA, mechanism of RLI has contsructed on the high perfusion pressures at inadequately perfused lesion sites after BPA. Feinstein et all. has drawn attention to the correlation between pre-op mPAP>35 mmHg and RLI rates (6). According to that epoprostenol administration (7), proflactic methylprednisolone use before procedure (3) and applying NIPPV after BPA (9,10) have studied to avoid RLI but they have not shown any significant difference. Therefore, strategy for avoiding complications has shifted to modifications of BPA techniques. For avoiding the reperfusion edema: 1-Procedure was restricted within 4-5 branches in a procedure. 2-Initial session, BPA performed for maximum 2 targeted vessels in a single lobe of the unilateral lung 3-BPA repeated 4-5 times in each lesion site. 4-Second intervention should be planned after 4-15 days of rest, and if it was necessary, additional intervention has been done after 12-16 weeks until reached the target- decreasing the mPAP by < 35 mmHg (7). With the increasing experience of operators, decline of complication rate to 36,3% by actualizing modifications routinely, has demonstrated with a multicenter registry (11). This results evoked that main cause of RLI would not be reperfusion itself. There was a learning curve effect and underlying cause of lung injury was vascular injury during procedures. So careful manipulation and refrain deep insertion of low tip guidewire, preferring small size of balloons, administering contrast agent gently has became the corner stones of BPA.
In our case we believed that the pulmonary injury occurred cause of more complex nature of the lesion. Even though we paid attention for other steps of the procedure, type of the lesion has been leaded deep insertions involuntarily. It was reported that success rates of BPA depend on lesion type. Pouching lesions’ success was reported less than 50% while ring-like stenosis, webs, abrupt vascular narrowing lesions’ was nearly 100% (12). Oppositely complication rate with ring-like stenosis, webs, abrupt vascular narrowing is 2%, 16% with subtotal obstruction type and 10% with pouching defects (12).
On the other hand, it is hard to compress organized thrombus with balloon dilatation. We know the information obtained from pathological examinations after BPA, incision occurs on the organized thrombus (13). Autopsy examinations has proved that dissection at tunica media leads partial detection of thrombus from vessel wall (14). So the pressure enlarges the vessel instead of crushing the thrombus between balloon and vessel. Ultimately we aim to increase lumen size by forcing thrombus into vessel wall with the help of dissection and wall thinning at lesion site irrespective of thrombus compression (15). However, using bigger size of balloons to enlarge lumen will facilitate lung injury. In our protocol, if the patient’s mPAP >40 mmHg, we prefer to start with 1,5 mm low profiled balloons, not to exceed 2 mm diameter balloon for initial session and inflate it with care of not to exceed 90% of the lumen dimension. We prefer bigger size of balloons for following sessions.
Another key point is keeping the team and medical staff alert for any complications that might develop during and after procedure. New developed desaturation (>4% decrease in saturation), cough, hemosputum should cause suspicion about complication. RLI can be diagnosed with bloody sputum, desaturation with infiltration in chest X-ray and newly developed shadow in computerized tomography (CT) images. Vascular complications can be detected by angiography with the clinic signs of chest pain, cough or hemoptysis. In our case even though we have confirmed no contrast extravasation during BPA, no hemosputum, no exceeding hypoxia, no pulmonary changes with chest X-ray after BPA, progressive tachypnea and desaturation occurred 20 minutes after procedure. As in our case most of RLI complications can successfully be managed with supplemental oxygen or NIPPV. For severe injury mechanical ventilation and percutaneous cardiopulmonary support may be required (15). Role of diuresis, steroid therapy or spironolactone analogues at RLI is not clear. Intensive care and monitorisation should be continued till the injury disappears from CT images. Treatment for vascular injury can be initiated with prolonged low-pressure balloon inflation proximal to the perforation. Albeit, prolonged balloon inflation might be insufficient for providing hemostasis, transcatheter coil embolization or covered stent placement might be required. (16)
PEA should be the first choice for appropriate CTEPH patients (2). Hybrid approach (first PEA than BPA) could be rescue therapy for residual PH after PEA. Bridging medical therapy before PEA or BPA is not clear. On the other hand, BPA is an alternative treatment for inoperable, peripheral type lesions. But it is still unclear how to treat very distal arteries with a diameter <100 µm, that behaves more likely idiopatic pulmonary arterial hypertension (13).
Conclusion:
BPA is an alternative treatment for inoperable CTEPH patients. However the intervention has some potential complications due to pulmonary vascular bed reperfusion injury, catheter manipulations, lesion based and technique related. Despite the factors that we can not change like lesion type, we have to be more focus on modifiable factors as catheter and balloon choice, contrast injection speed, restricting the number of balloon inflations to one or two pulmonary vascular segments per session.
References:
1. Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, Lang I, Madani MM, Ogino H, Pengo V, Mayer E. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62 (suppl 25):D92D99. doi: 10.1016/j.jacc.2013.10.024.
2. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y,Asteggiano R, Paolo Badano L, Albert Barberà J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol Ç, Falk V, Funck-Brentano C,Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Völler H, Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation(ISHLT). Eur Heart J. 2016;37:67119. doi: 10.1093/eurheartj/ehv317.
3- Thistlethwaite PA, Kaneko K, Madani MM, Jamieson SW. Technique and outcomes of pulmonary endarterectomy surgery. Ann Thorac Cardiovasc Surg. 2008;14:274282.
4- Lewczuk J, Piszko P, Jagas J et al. Prognostic factors in medically treated patients with chronic pulmonary embolism.Chest 2001; 119: 818823.
5- Voorburg JA, Cats VM, Buis B, Bruschke AV. Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism.Chest. 1988;94:12491253.
6- Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103:1013.
7- Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv.2012;5:748755. doi:10.1161/CIRCINTERVENTIONS.112.971077.
8. Adams A, Fedullo PF. Postoperative management of the patient undergoing pulmonary endarterectomy. Semin Thorac Cardiovasc Surg. 2006;18:250256.
9. Delclaux C, L’Her E, Alberti C, Mancebo J, Abroug F, Conti G, Guérin C, Schortgen F, Lefort Y, Antonelli M, Lepage E, Lemaire F, Brochard L.Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: A randomized controlled trial. JAMA. 2000;284:23522360.
10. Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care. 2006;10:R79.
11-Ogawa A, Matsubara H. Balloon Pulmonary Angioplasty: A Treatment Option for Inoperable Patients with ChronicThromboembolic Pulmonary Hypertension. Front Cardiovasc Med. 2015 Feb 17;2:4. doi: 10.3389/fcvm.2015.00004. eCollection 2015.
12- JCS Joint Working Group. Statement for balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension (JCS 2014). [In Japanese] Circ J
13-Ogawa A, Kitani M, Mizoguchi H, Munemasa M, Matsuo K, Yamadori I, et al. Pulmonary microvascular remodeling after balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Intern Med (2014) 53(7):72933. doi:10.2169/internalmedicine.53.1343
14- Kitani M, Ogawa A, Sarashina T, Yamadori I, Matsubara H. Histological changes of pulmonary arteries treated by balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv (2014) 7:8579. doi:10.1161/CIRCINTERVENTIONS.114.001533
15-Ogawa A, Matsubara H: Balloon pulmonary angioplasty: a treatment option for inoperable patients with chronic thromboembolic pulmo- nary hypertension. Front Cardiovasc Med 2:4, 2015
16- Ejiri K, Ogawa A, Matsubara H. Bail-out technique for pulmonary artery rupture with a covered stent in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv. 2015 Apr 27;8(5):752-3. doi: 10.1016/j.jcin.2014.11.024.
Figures:
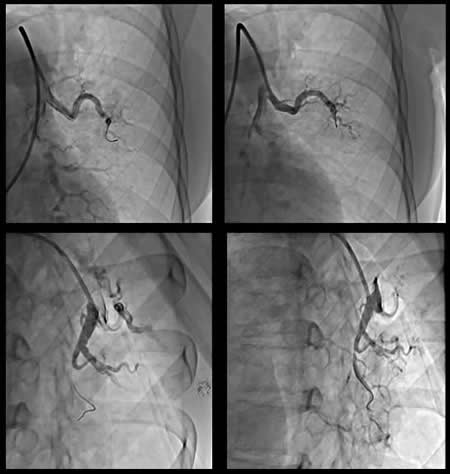
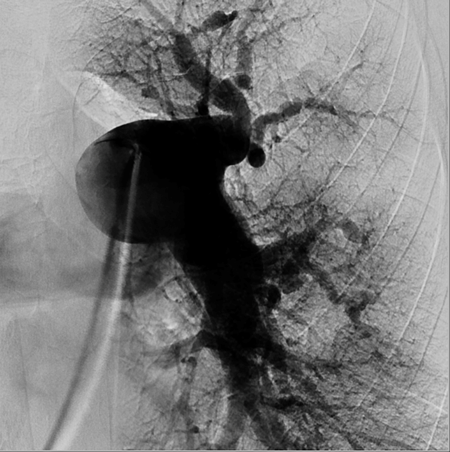
Figure-1: Angiografic views and DSA image after BPA sessions
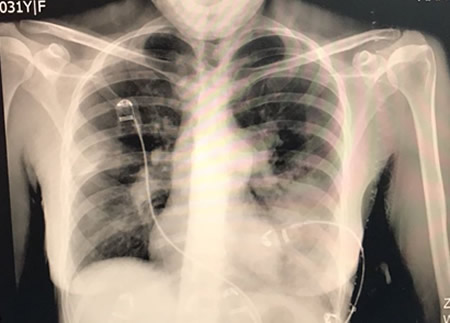
Figure-2: Chest X-ray after BPA sessions
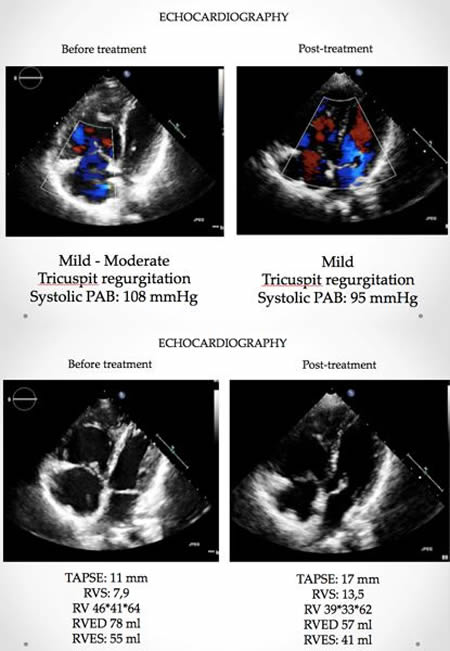
Figure-3: Echocardiography before and after treatment

|



